
This page titled The Order of Filling 3d and 4s Orbitals is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Jim Clark. Therefore the F electron configuration will. The remaining five electrons will go in the 2p orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital.
CA ELECTRON CONFIGURATION FULL
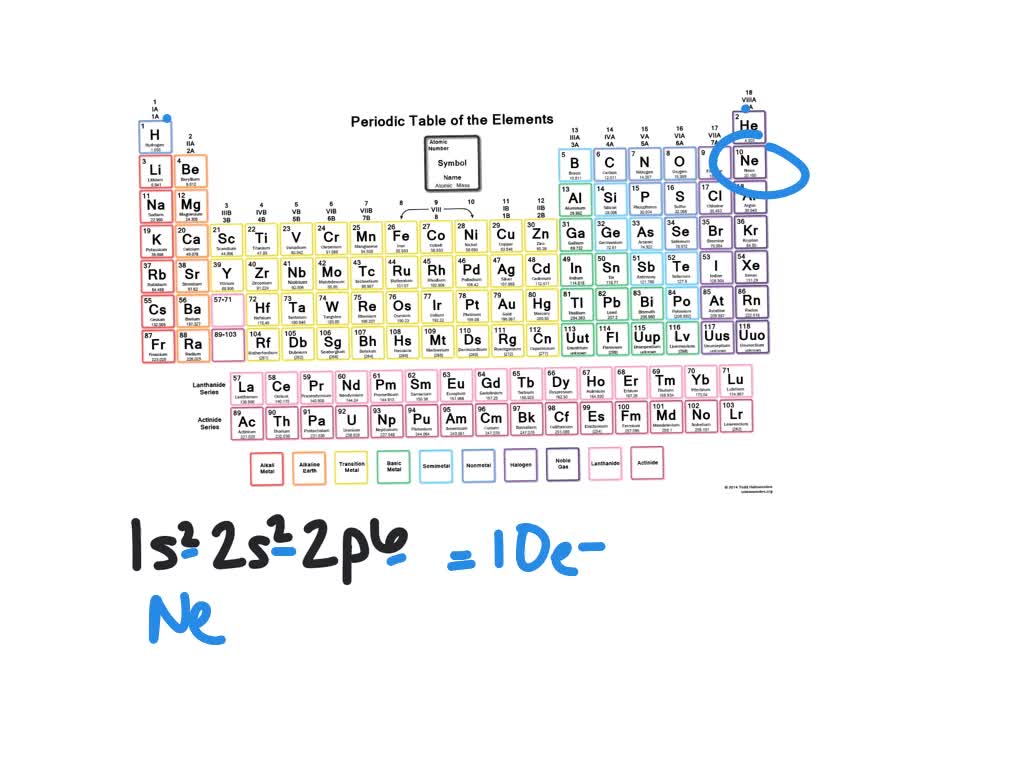
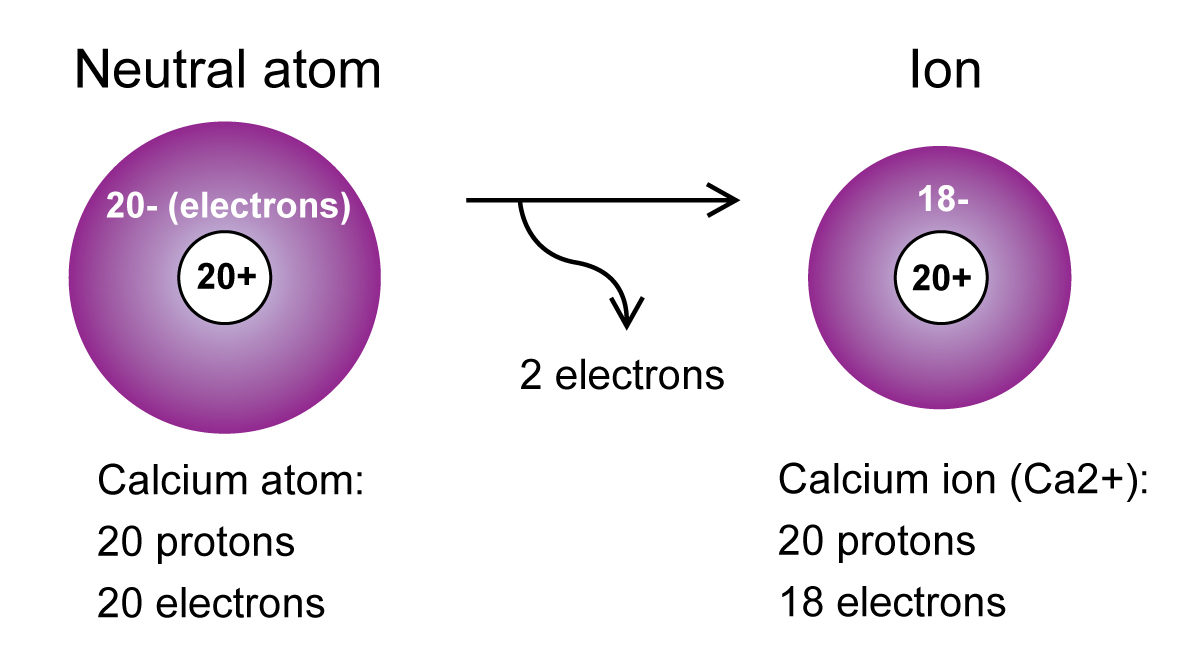
Mårtensson, "Core-Level Binding Energies in Metals," J. Eugen Schwarz: The Full Story of the Electron Configurations of the Transition Elements: Journal of Chemical Education, Vol. Fluorine is the ninth element with a total of 9 electrons. Lide, (Ed.) in Chemical Rubber Company handbook of chemistry and physics, CRC Press, Boca Raton, Florida, USA, 81st edition, 2000. Ley, Eds., Photoemission in Solids I: General Principles (Springer-Verlag, Berlin) with additional corrections, 1978. Calcium X-ray photoelectron spectra, calcium electron configuration, and other elemental information. Burr, "Reevaluation of X-Ray Atomic Energy Levels," Rev. They are tabulated elsewhere on the WWW (reference 4) and in paper form (reference 5). The data are adapted from references 1-3. I am grateful to Gwyn Williams (Jefferson Laboratory, Virginia, USA) who provided the electron binding energy data. The binding energies are quoted relative to the vacuum level for rare gases and H 2, N 2, O 2, F 2, and Cl 2 molecules relative to the Fermi level for metals and relative to the top of the valence band for semiconductors. All values of electron binding energies are given in eV. 1967, 47, 1300.Įlectron binding energies Electron binding energies for calcium. The electron configuration of Ca can also be represented using orbital filling diagrams as follows: Each orbital can contain two electrons with opposite spins. This makes it easier to understand and predict how atoms will interact to form chemical bonds.These effective nuclear charges, Z eff, are adapted from the following references: Calcium isotopes are 40 Ca, 42 Ca, 43 Ca, 44 Ca, 46 Ca, and 48 Ca. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The electron configuration of calcium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 Calcium is metal because it is in group 2 which makes it an alkaline earth metal and a powerful reducing agent. This give us the (correct) configuration of:įor the Cu+ ion we remove one electron from 4s1 leaving us with:įor the Cu2+ ion we remove a total of two electrons (one from the 4s1 and one form the 3d10) leaving us with Therefore, one of the 4s2 electrons jumps to the 3d9.
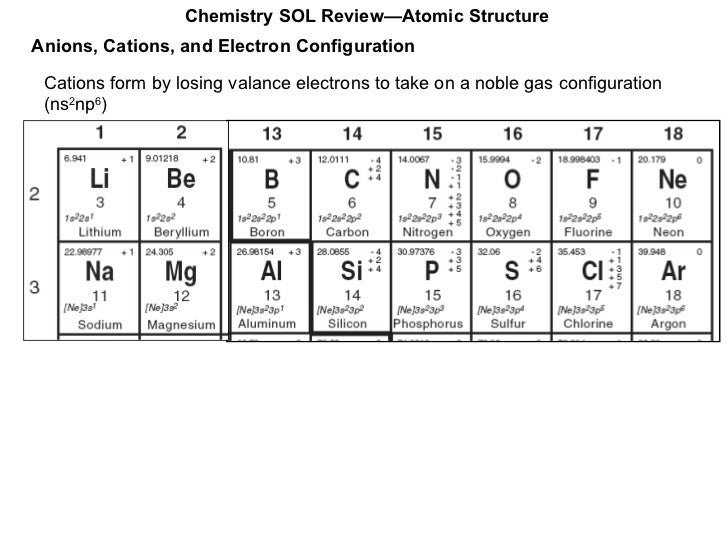
Half-filled and fully filled subshell have got extra stability. Therefore we have (still incorrect) 1s 22s 22p 63s 23p 63d 94s 2Ĭorrect Electron Configuration for Copper (Cu) Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written ( here is an explanation why).
Note that when writing the electron configuration for an atom like Cu, the 3d is usually written before the 4s. Therefore the expected electron configuration for Copper will be 1s 22s 22p 63s 23p 64s 23d 9. After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d9. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Elements in period 2 (building on the previous 2 electrons) need 8 more electrons to. Electron atomic and molecular orbitals A Bohr diagram of lithium. So elements in period 1 (hydrogen and helium) need 2 electrons to fill their valence shell. For example, the electron configuration of lithium, 1s☢s¹. We now shift to the 4s orbital where we place the remaining two electrons. Electron configurations describe where electrons are located around the nucleus of an atom. Since the 3s if now full we'll move to the 3p where we'll place the next six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. The p orbital can hold up to six electrons. The next six electrons will go in the 2p orbital. Since 1s can only hold two electrons the next 2 electrons for Copper go in the 2s orbital. In writing the electron configuration for Copper the first two electrons will go in the 1s orbital. Video: Cu, Cu +, and Cu 2+ Electron Configuration Notation


 0 kommentar(er)
0 kommentar(er)
